how to import medical equipment from china China Import and Export Fair Complex. Place your order with the vendor shipper or exporter and identify shipping terms that will be used.
How To Import Medical Equipment From China, 1Attend Chinese Trade Fairs Canton Import And Export Fair Global Sources Trade Show 2. HKTDC hosts several annual trade fairs in spring and autumn. At TIBA we help companies in the pharmaceutical and medical field import products such as medical instruments medical and surgical equipment medicines tattooing materials natural medicines.
 Government Proposes Health Cess On Import Of Medical Equipment The Economic Times From m.economictimes.com
Government Proposes Health Cess On Import Of Medical Equipment The Economic Times From m.economictimes.com
HKTDC hosts several annual trade fairs in spring and autumn. In addition just four countries namely Switzerland the US the UK and China provide between 65 medical equipment and 76 pharmaceuticals of EU imports. At TIBA we help companies in the pharmaceutical and medical field import products such as medical instruments medical and surgical equipment medicines tattooing materials natural medicines.
Profit from additional features with an Employee.
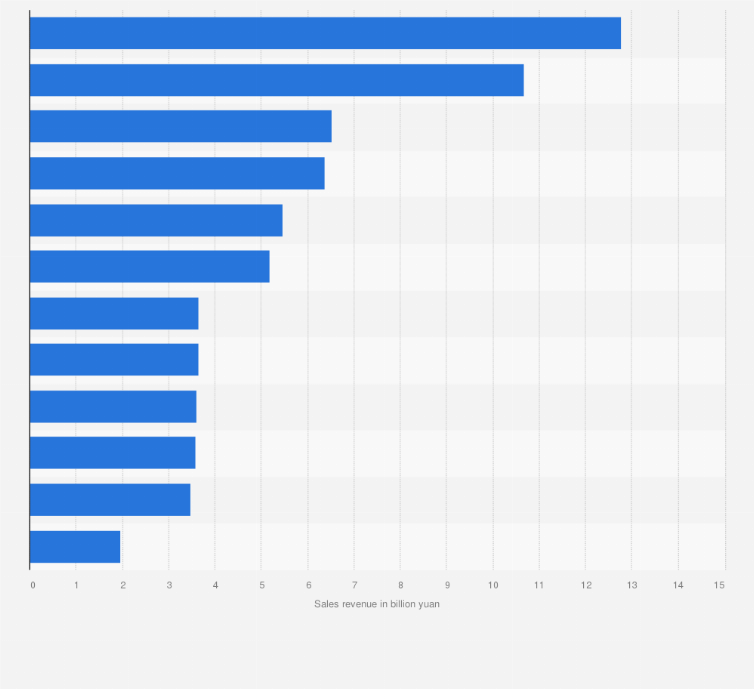
Value of medical equipment imports to China 2008-H1 2021. If a manufacturer wants to renew a devices registration a renewal application should be submitted 6 months prior to the expiration date to the same department that received the original registration submission. The most important thing is to find a good supplier from Chinabut howSome products are unpopular than hard to find the right supplierThere is no. Once you have selected your supplier request a PI Proforma Invoice or Quote Sheet for your prospective purchases to include the harmonized system number description value per item. Once the product has been registered and classified the importer has a choice of 2 options set out below.
Another Article :

If the company wishes to import the good and sell directly to the hospitals or any health facility then the company importer will need to be registered as an authorised reseller of the devices. However procuring medical supplies from China is becoming ever more complex and should be approached with caution. China was ranked as the fourth-largest supplier in 2018 accounting for 11 57 billion of the total. If a manufacturer wants to renew a devices registration a renewal application should be submitted 6 months prior to the expiration date to the same department that received the original registration submission. There are two types of import data one is IGM Import General Manifesto data which has limited details and the other one is import data which have complete details. Government Proposes Health Cess On Import Of Medical Equipment The Economic Times.
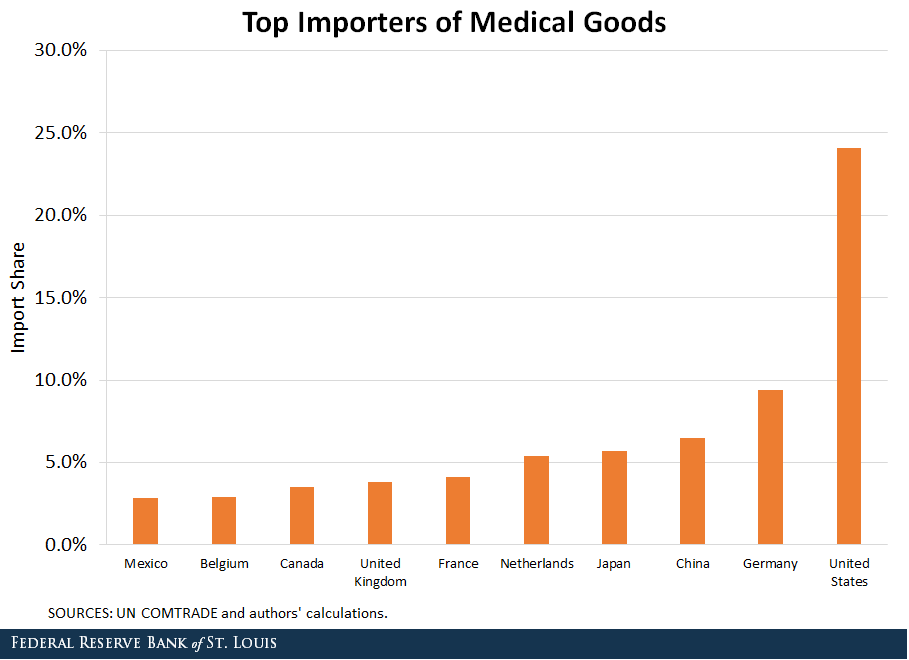
How to import medical equipment from China. Of course there are many other products that you can import from China. The following trade fairs are relevant to buyers of medical devices printing machinery and electrical machinery. Machinery and plant equipment Tricycles motorcycles and bicycles Steel products Ceramic items Electricals and electronic equipment Construction materials Vehicles Plastic and plastic products Paper Pharmaceuticals and medical equipment These are not the only ones. 1Attend Chinese Trade Fairs Canton Import And Export Fair Global Sources Trade Show 2. Protectionism And Dependence On Imports Of Essential Medical Equipment St Louis Fed.

China Import and Export Fair Complex. HKTDC hosts several annual trade fairs in spring and autumn. China was ranked as the fourth-largest supplier in 2018 accounting for 11 57 billion of the total. If a manufacturer wants to renew a devices registration a renewal application should be submitted 6 months prior to the expiration date to the same department that received the original registration submission. Place your order with the vendor shipper or exporter and identify shipping terms that will be used. Healthcare Resource Guide Rwanda.
You have to know the HS code of the electronic product to buy the import data. In each product category as few as five countries provide at least 72 of EU imports. As well as from multi-function beauty equipment. 1Attend Chinese Trade Fairs Canton Import And Export Fair Global Sources Trade Show 2. Value of medical equipment imports to China 2008-H1 2021. Exhibitor List China International Import Expo.

In each product category as few as five countries provide at least 72 of EU imports. Yeahthats very tricky if you want to go through import procedure yourselfOne cannot simply import medical equipment into China. Medical device registration in China is now valid for 5 years previously it was valid for only 4 years. Place your order with the vendor shipper or exporter and identify shipping terms that will be used. Instead importers or other entities may be required to register with the FDA. Protected At Home China S Medical Device Industry Looks Abroad Council On Foreign Relations.
And whether import medical equipment is class ii. Machinery and plant equipment Tricycles motorcycles and bicycles Steel products Ceramic items Electricals and electronic equipment Construction materials Vehicles Plastic and plastic products Paper Pharmaceuticals and medical equipment These are not the only ones. Importing and Exporting Medical Devices. Once the product has been registered and classified the importer has a choice of 2 options set out below. Given that China has emerged as one of the first countries able to bring its people out of lockdown now that the worst of the virus has passed there is significant demand for Chinese manufactured medical equipment outside of China. Us Reliance On Others For Medical Equipment Vox Cepr Policy Portal.
Yeahthats very tricky if you want to go through import procedure yourselfOne cannot simply import medical equipment into China. Place your order with the vendor shipper or exporter and identify shipping terms that will be used. A wide variety of import medical equipment options are available to you such as 1 year 2 years and 3 years. While import of medical equipment and tools from China surged overall import from China declined during the first seven months of this fiscal. Foreign establishments that manufacture medical devices andor radiation-emitting electronic products that are imported into the United States US must. China Largest Medical Device Companies 2021 Statista.

In each product category as few as five countries provide at least 72 of EU imports. Dollar as of first seven months of this fiscal. Some entities may also be required to list all of the devices and activities performed. The most important thing is to find a good supplier from Chinabut howSome products are unpopular than hard to find the right supplierThere is no. Chinese suppliers featured prominently in the patient aids sector accounting for almost a quarter of the import total. China S Fast Growing Medical Device Market Presents Huge Opportunities For Foreign Firms.
Once you have selected your supplier request a PI Proforma Invoice or Quote Sheet for your prospective purchases to include the harmonized system number description value per item. Instead importers or other entities may be required to register with the FDA. China Import and Export Fair Complex. You have to know the HS code of the electronic product to buy the import data. Importing and Exporting Medical Devices. Us Reliance On Others For Medical Equipment Vox Cepr Policy Portal.
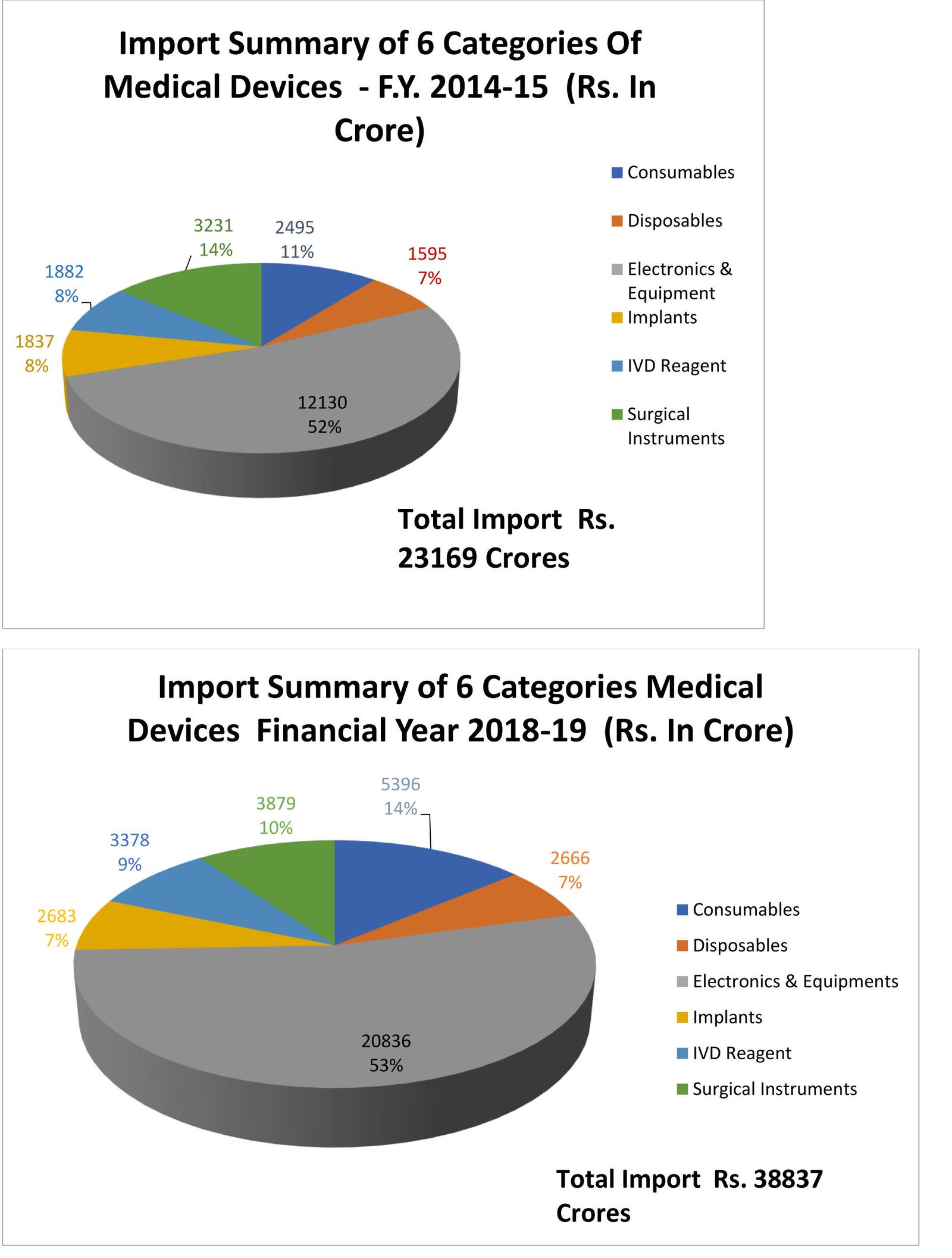
At TIBA we help companies in the pharmaceutical and medical field import products such as medical instruments medical and surgical equipment medicines tattooing materials natural medicines. China was ranked as the fourth-largest supplier in 2018 accounting for 11 57 billion of the total. At TIBA we help companies in the pharmaceutical and medical field import products such as medical instruments medical and surgical equipment medicines tattooing materials natural medicines. Hong Kong Trade and Development Council HKTDC Fairs. And whether import medical equipment is class ii. Import Of Medical Devices Up By Record 24.

China was ranked as the fourth-largest supplier in 2018 accounting for 11 57 billion of the total. How to import medical equipment from China. HKTDC hosts several annual trade fairs in spring and autumn. Place your order with the vendor shipper or exporter and identify shipping terms that will be used. If there is no wooden packaging you must declare non-wood packaging. Manufacturing Medical Devices Covid 19.
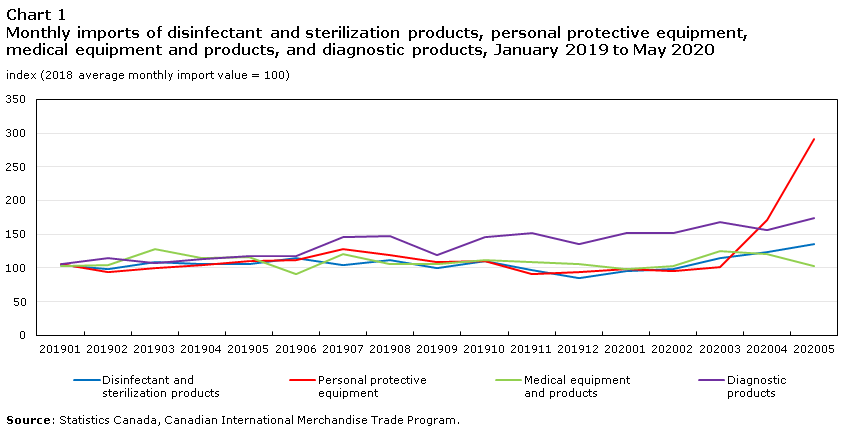
Place your order with the vendor shipper or exporter and identify shipping terms that will be used. If the company wishes to import the good and sell directly to the hospitals or any health facility then the company importer will need to be registered as an authorised reseller of the devices. If there is no wooden packaging you must declare non-wood packaging. Online China Supply Wholesale Market. Given that China has emerged as one of the first countries able to bring its people out of lockdown now that the worst of the virus has passed there is significant demand for Chinese manufactured medical equipment outside of China. Trade In Medical And Protective Goods May 2020.

Some entities may also be required to list all of the devices and activities performed. While import of medical equipment and tools from China surged overall import from China declined during the first seven months of this fiscal. Some entities may also be required to list all of the devices and activities performed. Imported medical devices valued at 198 billion from the EU-28 in 2018 equal to 38 of the total. Import precautions goods from China A. Global Medical Trade In The Time Of The Coronavirus Pandemic Equitable Growth.

If the company wishes to import the good and sell directly to the hospitals or any health facility then the company importer will need to be registered as an authorised reseller of the devices. China was ranked as the fourth-largest supplier in 2018 accounting for 11 57 billion of the total. The following trade fairs are relevant to buyers of medical devices printing machinery and electrical machinery. In each product category as few as five countries provide at least 72 of EU imports. There are two types of import data one is IGM Import General Manifesto data which has limited details and the other one is import data which have complete details. Healthcare Resource Guide Nigeria.

An import health licence issued by the Spanish Agency of Medicines and Medical Devices AEMPS is required in order to import medical devices and products. The following trade fairs are relevant to buyers of medical devices printing machinery and electrical machinery. Online China Supply Wholesale Market. Medical device registration in China is now valid for 5 years previously it was valid for only 4 years. You can also choose from online technical support return and replacement and free spare parts. Coronavirus China Bans Two Medical Equipment Exporters For Tarnishing The Country S Image South China Morning Post.










